Table Of Content
- A New Approach to Treating Genetic Disease
- Design Therapeutics Highlights Upcoming Milestones and Reports Second Quarter 2022 Financial Results
- MarketBeat Products
- Data to be Presented during the 2021 Virtual Myotonic Dystrophy Foundation Annual Conference
- BML Capital Management LLC Invests $2.73 Million in Design Therapeutics, Inc. (NASDAQ:DSGN)
- Bristol Myers outlines ‘about 12’ pipeline cuts as it executes major cutbacks

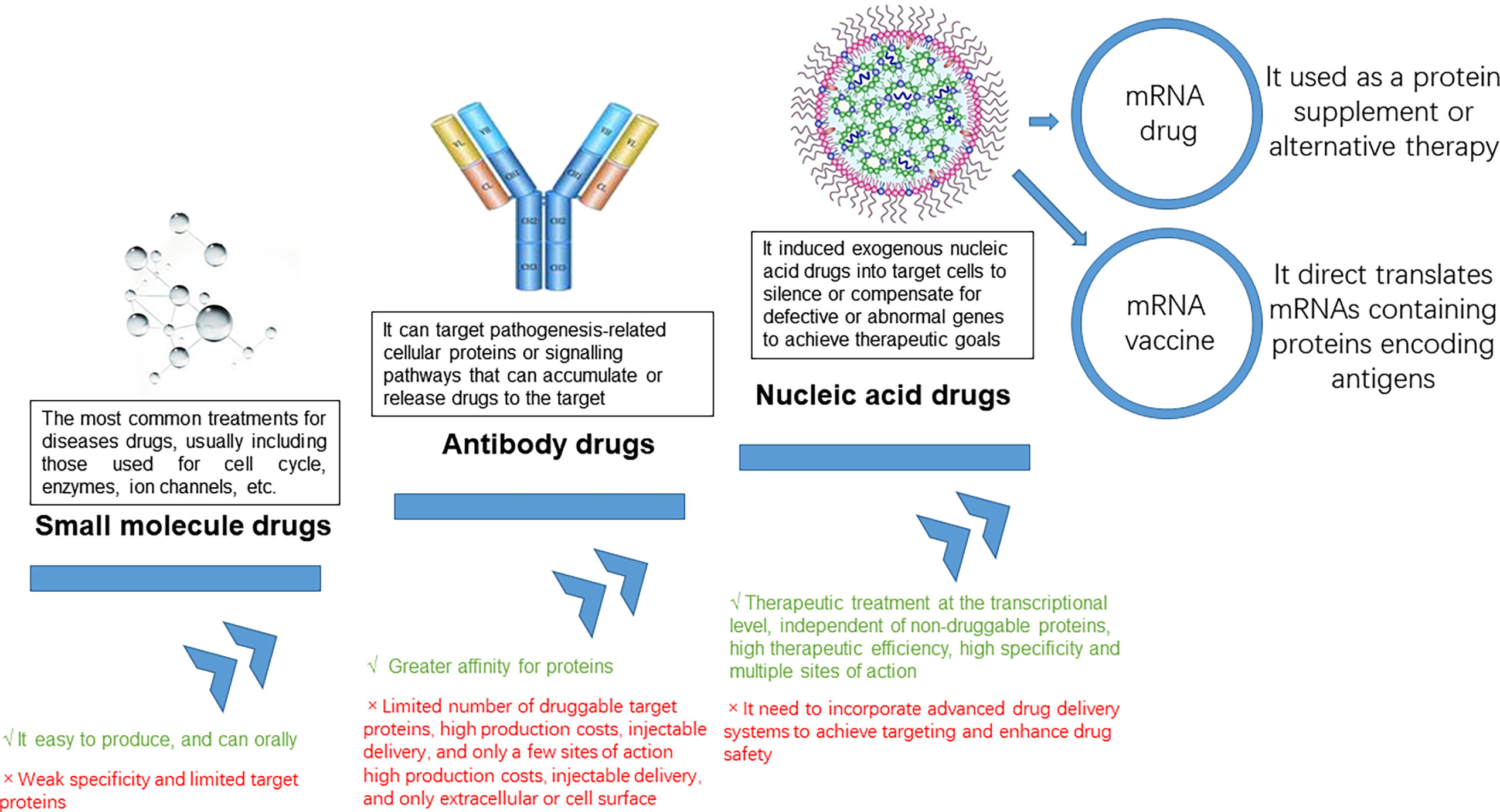
In addition to its lead GeneTAC™ small molecule, DT-216, in development for patients with Friedreich ataxia, the company is advancing programs in Fuchs endothelial corneal dystrophy, Huntington’s disease and myotonic dystrophy type-1. We are developing a portfolio of GeneTAC™ product candidates designed to address genetic diseases driven by inherited nucleotide repeat expansions. GeneTAC™ molecules are designed to be a novel class of disease-modifying small molecule therapeutic candidates that can either dial up or dial down the expression of a specific disease-causing gene to address the underlying cause of disease. The development of drugs to treat genetic disease has largely focused on novel complex modalities such as gene therapy or gene editing that have proven to be challenging to develop. Until recently, scientists had not succeeded in using small molecules for selective, targeted transcription modulation. Through our GeneTAC™ platform, we have designed small molecules that effectively target and dial up or down the expression of specific genes, with one of our lead compounds having entered the clinic in 2022.
A New Approach to Treating Genetic Disease
There is currently no effective therapeutic intervention that addresses the root cause of the disease. Corneal transplantation, including various modalities of keratoplasty, is the only treatment option to correct advanced FECD. In August 2023, we reported data from the MAD Phase 1 clinical trial showing that DT-216 was generally well-tolerated and exhibited the ability to overcome the FXN transcription impairment that causes FA. We have developed a new drug product candidate, DT-216P2, for patients with FA that demonstrated an improved pharmacokinetic profile and a favorable injection site safety profile in nonclinical studies. Xaira is building significant AI research capabilities spanning fundamental computational methods development and their application to biological discovery, the design of drug-like matter, and clinical development.
Design Therapeutics Highlights Upcoming Milestones and Reports Second Quarter 2022 Financial Results
Affected individuals have reduced life expectancy, with many premature deaths caused by complications of cardiomyopathy at about the end of the fourth decade of life. The estimated prevalence of FA is 1 in 40,000–50,000, affecting more than 5,000 individuals living in the United States and more than 20,000 in Europe. Our team consists of recognized global leaders in the field of DNA-targeted small molecules, transcription modulation, medicinal chemistry, translation, and development into clinical applications. "We have reached the point where AI finally allows us to see biology in new ways, and translate those discoveries to better treatments for disease," said Robert Nelsen, Managing Director and Co-Founder of ARCH Venture Partners. "This creates an enormous opportunity for us to rethink drug discovery entirely. For this reason, Xaira is the largest initial funding commitment in ARCH history."
MarketBeat Products
These HD GeneTAC™ small molecules are designed to address the root cause of HD by selectively reducing the toxic mtHTT gene product and distribute to the whole brain when administered systemically. The research collaboration was established to design and test agonistic antibodies targeting tumor necrosis factor receptor type II (TNFR2), a novel, biologically-validated disease target implicated in a wide range of autoimmune diseases that has been difficult to drug with conventional medicines. “At Design, our vision is to develop a portfolio of first or best-in-class small molecules capable of treating a host of degenerative diseases by working with a patient’s natural genome to restore cellular health.
From the University of Pennsylvania School of Medicine and an MBA (Mayer Scholar) from the Wharton School of the University of Pennsylvania. SAN FRANCISCO, April 23, (BUSINESS WIRE)--Xaira Therapeutics launched today on a mission to help re-engineer the way we discover and develop medicines through the end-to-end application of emerging AI technologies. A star-studded mix of venture capitalists and scientists, backed by more than a billion dollars, is launching an ambitious biotech that aims to reinvent drug R&D using artificial intelligence, the group exclusively told Endpoints News. (Steve) MayoBren Professor of Biology and Chemistry; Merkin Institute ProfessorThe focus of the lab is the coupling of theoretical, computational, and experimental techniques for the study of structural biology. Click the link below and we'll send you MarketBeat's list of ten stocks that will drive in any economic environment. While Design Therapeutics currently has a "Hold" rating among analysts, top-rated analysts believe these five stocks are better buys.
BML Capital Management LLC Invests $2.73 Million in Design Therapeutics, Inc. (NASDAQ:DSGN)
The variability of results observed with this current method substantially limited the utility of FXN protein measurements to assess DT-216 pharmacology. Myotonic dystrophy (DM1) is a monogenic, autosomal dominant, progressive neuromuscular disease that affects skeletal muscle, heart, brain and other organs. The cardinal features include muscle weakness, myotonia (slow muscle relaxation) and early cataracts. In addition, affected individuals often experience cardiac arrhythmias and changes in neuropsychological function.
Fuchs Endothelial Corneal Dystrophy Program
Aseem Z. Ansari, Ph.D. is a co-founder and scientific advisor of Design Therapeutics. Dr. Ansari pioneered the development of synthetic transcription factors, and over the past 25 years, performed the foundational research that led to Design Therapeutics. Dr. Ansari is the chair of the Department of Chemical Biology and Therapeutics at St. Jude Children’s Research Hospital. He received his Ph.D. in chemical biology from Northwestern University and was a postdoctoral fellow at Harvard and MIT. Dr. Ansari’s research has produced important new insights into cellular processes that govern gene expression and has developed novel small molecules that can influence gene regulatory networks and cell fate.
Design Therapeutics Company Profile
Prior to Design, Ms. Burgess served as the senior vice president of finance at Otonomy, Inc., Corporate Controller at Apricus Biosciences, and a consultant to several other biopharmaceutical companies. Ms. Burgess is a licensed CPA and completed her MBA at San Diego State University. This collective knowledge and expertise serve as the engine for our innovative approach and make us uniquely equipped to harness the power of our GeneTAC™ small molecules for the treatment of genetic disease. DT-216 Program Next StepsThe initial results from Design’s Phase 1 multiple ascending dose trial underscore the promise of DT-216 as a potential disease-modifying treatment for FA. FXN protein measurements in skeletal muscle had substantial overlap between FA patients and carriers and showed high intra-individual variability. More than half of FA patients had 25% or more variation in FXN protein levels between visits, with intra-individual coefficient of variation of 69%.
Bristol Myers outlines ‘about 12’ pipeline cuts as it executes major cutbacks
Corton Capital Inc. now owns 27,134 shares of the company's stock worth $64,000 after purchasing an additional 9,811 shares in the last quarter. Algert Global LLC boosted its position in shares of Design Therapeutics by 83.3% during the third quarter. Algert Global LLC now owns 35,620 shares of the company's stock worth $84,000 after acquiring an additional 16,190 shares during the last quarter.

About Design TherapeuticsDesign Therapeutics is a clinical-stage biotechnology company developing a new class of therapies based on its platform of GeneTAC™ gene targeted chimera small molecules. The company’s GeneTAC™ molecules are designed to either dial up or dial down the expression of a specific disease-causing gene to address the underlying cause of disease. In addition to its lead GeneTAC™ small molecule, DT-216, in development for patients with Friedreich ataxia, the company is advancing programs in Fuchs endothelial corneal dystrophy and myotonic dystrophy type-1. Discovery efforts for multiple other serious degenerative disorders caused by nucleotide repeat expansions are also underway, including for fragile X syndrome, spinocerebellar ataxias, Huntington disease, spinobulbar muscular atrophy, and C9orf72-amyotrophic lateral sclerosis/frontotemporal dementia. Design’s lead program is focused on the treatment of Friedreich ataxia, followed by a program in myotonic dystrophy type-1 and discovery efforts for multiple other serious degenerative disorders caused by nucleotide repeat expansions. About Design TherapeuticsDesign Therapeutics is a biotechnology company developing a new class of therapies based on its platform of GeneTAC™ gene targeted chimera small molecules.
GeneTAC™ molecules are also designed to work with the natural genome without altering a patient’s DNA. Dr. Louise Rodino-Klapac discusses the science of limb-girdle muscular dystrophies (LGMDs) and how our gene therapy engine will help engineer potential therapies for this group of inherited neuromuscular diseases. Royal Bank of Canada restated a "sector perform" rating and set a $4.00 target price on shares of Design Therapeutics in a research report on Wednesday, March 20th. Wedbush restated a "neutral" rating and set a $5.00 target price on shares of Design Therapeutics in a research report on Wednesday, March 20th. Seven analysts have rated the stock with a hold rating, According to MarketBeat.com, the stock currently has a consensus rating of "Hold" and a consensus price target of $5.50.
Design has since shown that an improved formulation had favorable injection site tolerability following multiple intravenous administrations and enabled dosing to increase tissue exposure. Our FECD program leverages our expertise in designing GeneTAC™ small molecules that address the underlying cause of the disease. FECD GeneTAC™ molecules have shown to markedly reduce nuclear foci and improve spliceopathy in FECD CEC cultures derived from donors who underwent corneal transplant.In December 2022, DT-168 was declared a drug candidate for FECD. DT-168 is a GeneTAC™ small molecule designed to target the CTG repeats in the TCF4 gene and selectively block transcription of the expansion-containing allele. He has deep experience developing innovative therapies across multiple diseases and therapeutic modalities through all phases of drug development. Prior to joining Design, Dr. Kim was Chief Medical Officer of Avidity Biosciences.
Most recently, Dr. Lappe served as executive chairman of the board of directors for Mirati Therapeutics (MRTX), a role he had served at until he resigned in February 2019, after becoming a member of the board in June 2012 and chairman of the board in July 2013. Previously, Dr. Lappe was group senior vice president, Pfizer Worldwide Research and Development and chief scientific officer for CovX in San Diego. Dr. Lappe has also served as vice president for cardiovascular and metabolic diseases at Pharmacia where he was also site leader for Pharmacia in St. Louis. Prior to joining Pharmacia, he held positions with Wyeth, Rorer Central Research, CIBA Geigy and Searle Pharmaceuticals. "In my previous roles, I witnessed an earlier generation of technologies fundamentally change drug discovery, delivering new medicines that alleviate the burden of disease for many patients," said Dr. Marc Tessier-Lavigne, CEO.
Design Therapeutics, Inc. (DSGN) Upgraded to Buy: Here's What You Should Know - Yahoo Finance
Design Therapeutics, Inc. (DSGN) Upgraded to Buy: Here's What You Should Know.
Posted: Mon, 18 Dec 2023 08:00:00 GMT [source]
Prior to Avidity, he served as Clinical Research Head, Vice President of Clinical Development and Chair of the Clinical Trial Review Board at Alnylam Pharmaceuticals. Before Alnylam, he served in roles of increasing responsibility in global development at MyoKardia, Inc. and Amgen. He contributed substantially to the development and/or approvals of Givlaari® (givosiran), Amvuttra® (vutrisiran), Leqvio® (inclisiran), Camzyos® (mavacamten), Corlanor® (ivabradine), and Repatha® (evolocumab). "In starting Xaira, we have brought together incredible multidisciplinary talent and capabilities at the right time to reimagine our entire approach, from drug discovery to clinical development." These data, supplemented by a growing body of data from the company’s GeneTAC program for Friedrich ataxia, support the continued advancement of the DM1 program and rationale to evaluate the utility of the GeneTAC approach in multiple additional nucleotide repeat expansion diseases. Clinical results from our lead GeneTAC™ small molecule (DT-216) for Friedreich ataxia have shown that our GeneTAC™ approach can modulate target gene expression in patients after a single dose, demonstrating the mechanism and promise of this platform.








.jpg)